Amazing Tips About How To Tell If An Element Is Positive Or Negative

An element is an example of an atom with a fixed number of positive protons within the nucleus.
How to tell if an element is positive or negative. Result 2 answers. If the number of protons is higher than electrons then its positivley charged. Result the atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser electronegativity acquires a.
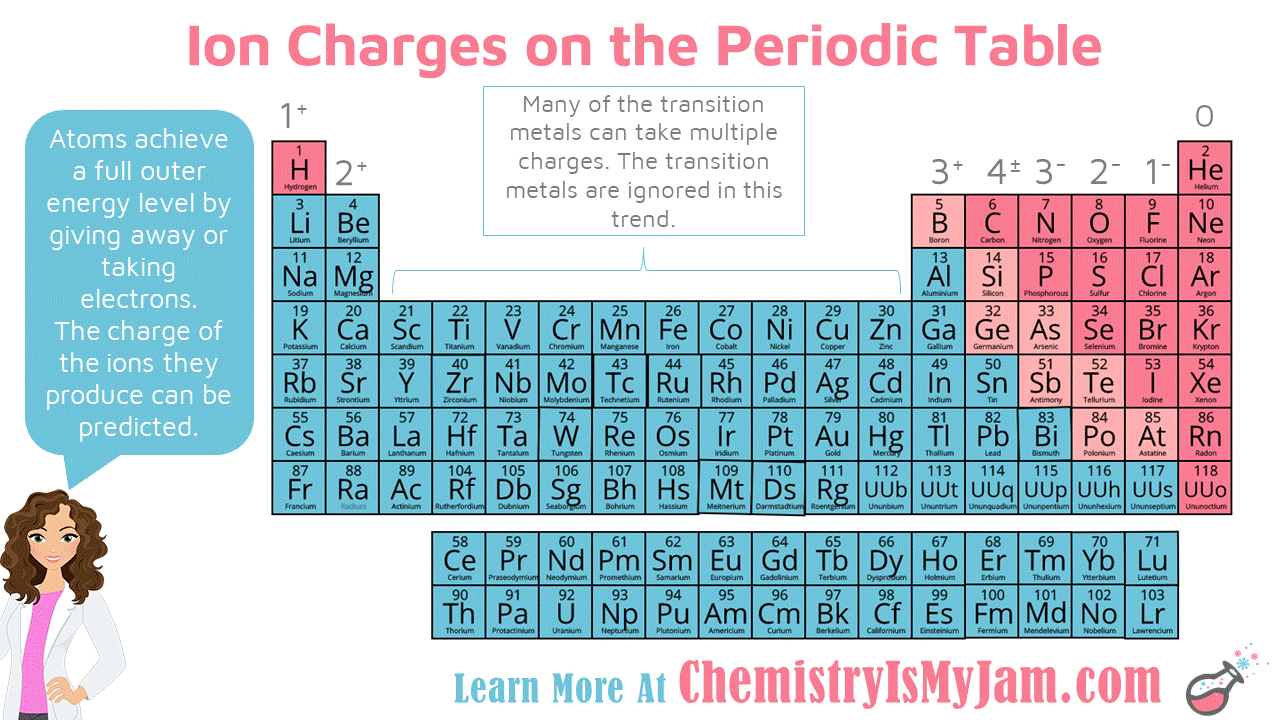
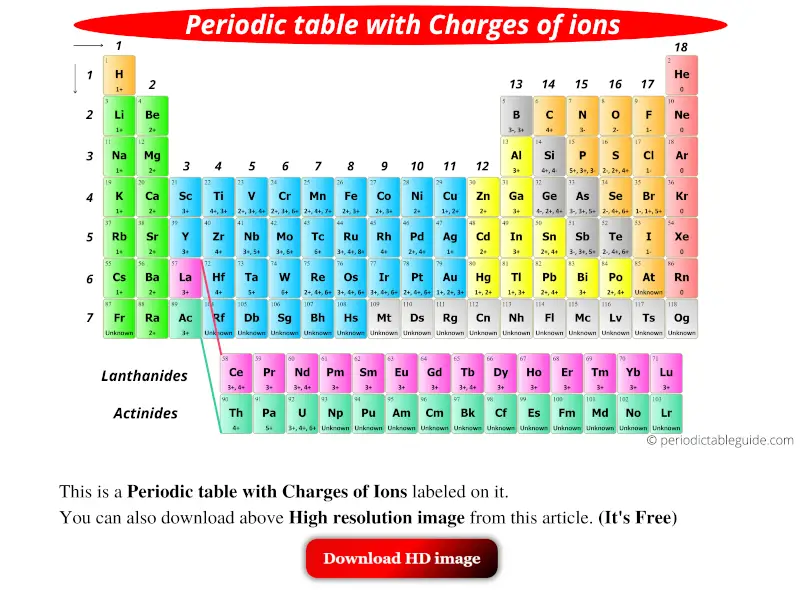
Result moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Result if you look at the periodic table, you might notice that elements on the left side usually become positively charged ions (cations) and elements on. If the magnitude of the charge is greater than 1, then the number is written before the +.
Anions are ions that are negatively. Compare with known positive/negative charge. Result recall that a lowercase greek delta ( δ δ) is used to indicate that a bonded atom possesses a partial positive charge, indicated by δ+ δ +, or a.
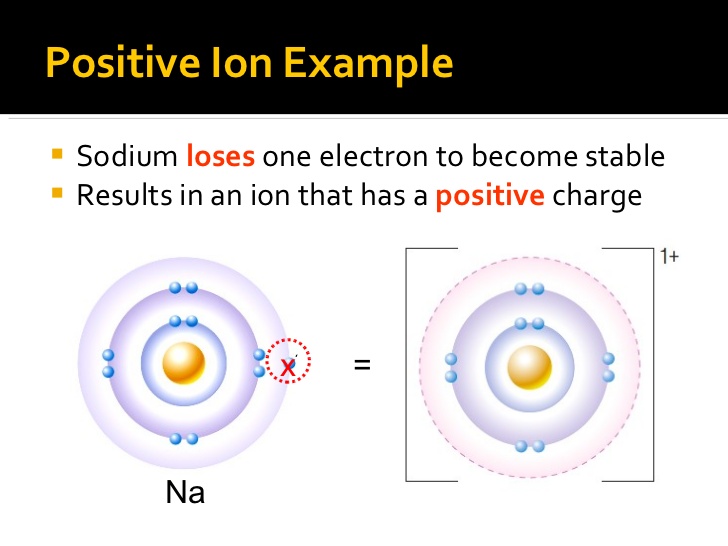
Result 93 rows there are four ways to find the charge of an element: Working out the charges of ions. For example, sodium is an element with 11 protons within the nucleus and 11 electrons.
Result if the charge is a single positive or negative one, the number 1 is not written; Atoms are composed of three particles: Can be worked out using patterns in the.
Result remove from my bitesize. How do we determine if a charge is positive, in this question? The usual charge of an element is common to its group.
Electronegativity of an element is the ability to. Electropositive character means the tendency of an atom to lose e− and form a positive ion. For example, if an element has six protons but.
Result utilizing ionic properties. Result an atom becomes charged when the number of protons does not equal the number of electrons. Find some material which you know takes up or gives away electrons.
Positive or negative, the charge and rod are there. In order to tell the sign of an object charge, you need another object with a known positive or negative charge. Result the ions are positive, because they have more protons close proton subatomic particle with a positive charge and a relative mass of 1.
If the atom has more electrons than protons, it is a negative ion, or anion. Result how do you know if a chemical is positive or negative? The nucleus is composed of protons and neutrons, collectively referred.


.PNG)



:max_bytes(150000):strip_icc()/cheat-sheet-positive-negative-numbers-23125190-v3-FINAL-5c5db3d2c9e77c000159c36a.gif)